Today, we live amidst a viral pandemic, COVID-19. In more ways than one, so much of our world has changed. COVID-19 has taught us that a small virus can almost shut the world’s social and economic machinery down. It is certainly a phenomenon the world was not prepared for.
This case study talks about yet another possible pandemic and what we can do to circumvent this. It is caused by bacteria which can survive for long periods outside the body unlike the coronavirus. Thereby, causing it to spread through food, air and water. As a result, social distancing would be ineffective and one can only imagine its magnitude. Speaking of which, AMR kills about 700,000 individuals annually and if left untreated, it is estimated to take away 10 million lives by the year 2050. This is more lives than cancer and is a greater threat to human life than COVID-19.

WHO-Director General, Tedros Adhanom stated that Antimicrobial resistance (AMR) is a “slow tsunami that threatens to undo an entire generation of medical progress.” This is because bacterial infections cause common surgeries that have been performed for decades very risky. Healthy individuals who may undergo normal surgeries such as C-sections, joint replacements or even tooth extractions are at a high risk of death. They may contract fatal bacterial infections from the site of surgery, since, we may not have the antibiotics to treat such infections.
AMR- A Global Problem
The hotspots of resistant bacteria are places where there are poor hygiene systems and insufficient waste management. This could be residues from our homes or hospitals or pharmaceutical manufacturing sites. These residues seep into water and soil forcing normal bacteria in such environments to develop genes that resist these antibiotics. Such bacteria are called ‘Superbugs’ and these resistance genes are like their superpowers.
One of the many unique things about bacteria is that they love sharing their superpower resistance genes even with other unrelated bacteria. This inter-species transfer of resistance genes continues to happen even after the antibiotic is removed from the environment. This means that there is a strong need to control antibiotic waste at source.
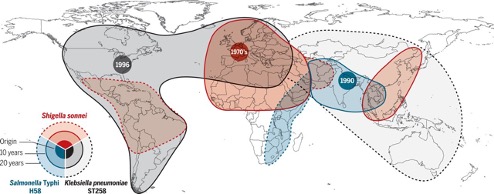
It is true that a handful of countries such as the Nordics have largely mastered waste management. However, much like the corona virus, resistant bacteria in one part of the world can rapidly spread to another part in just a couple of hours when someone takes a flight or when someone sends a parcel or sometimes even through migratory birds. (Fig 3) This is because bacteria can survive a wide variety of harsh environmental conditions even outside the body by switching to a low energy state. A state in which some bacteria remain undetectable by routine quality control methods. So, by and large, this is an urgent global problem that needs a collaborative solution.
Lack of pharma investment in the antibiotics space
One may ask why pharma companies have not come with new antibiotics. The answer lies in the game-changing play of profit and loss. According to a 2017 estimate, the cost of bringing an antibiotic to the market is about US$1.5 billion. However, the revenue generated by the sale of antibiotics is roughly US$ 46 million per year. Also, governments in the developed world try to keep the price low and physicians do not prescribe large volumes of antibiotic to prevent further build-up of resistance.
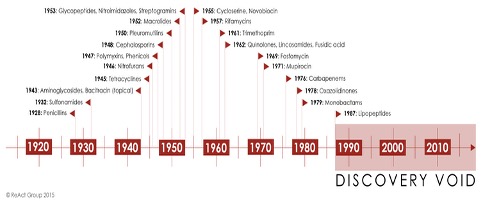
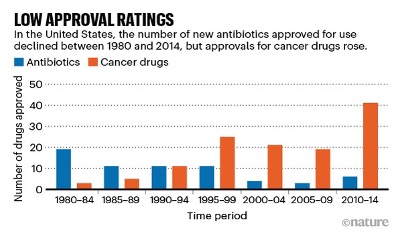
These price and volume factors result in diminishing profits and have resulted in decreased investment in the antibiotics space and poor replenishment of the antibiotic pipeline. As a result, pharma companies are choosing to invest in other profitable sectors such as cancer (Fig 4). The world has not seen a new class of antibiotic since 1987 (Fig 3)
Building a more resilient world
Below is a brief glance on how Antimicrobial resistance (AMR) can be controlled in various sectors.
Let us craft more solutions together to build a more resilient world with NAI’s unique case study programmes.
Event:
Large-scale overuse of antibiotics by the public even for non-bacterial infections.
Possible root causes:
- Lack of sufficient regulations in dispensing Over-the-Counter (OTC) bacterial antibiotics in developing economies such as India and Nepal.
Solutions:
- Government regulations that make declaring the number of OTC drugs sold available. So, governments can keep track of OTC drugs dispensed to the public.
- Policies that allow doctors to prescribe antibiotics on a need basis as much as possible. This could tackle the off-label public use of antibiotics for non-bacterial infections.
Event:
Overuse of antibiotics in the livestock sector.
Possible root causes:
- Animal feed contains growth-promoting antibiotics for better meat yields
- Rapid spread of bacterial Infections from unavoidable external sources such as workers or vehicles in animal farms necessitating the use of antibiotics.
Solutions:
- Governments or public-private partnerships to promote the use of alternate forms of food enzymes, fatty acids, essential oils and herbal extracts without growth-promoting antibiotics to help livestock companies achieve the same yield antibiotic-free.
- Improved approaches to hygiene such as quarantining of new animals. Also, mandating workers, vehicles and all other articles to use disinfectants before entering the farm.
- The use of cameras and artificial intelligence systems to track animal health with minimal human interference. So, there is less incidence of disease leading to less use of antibiotics.
References
- https://accesstomedicinefoundation.org/amr-benchmark/2020-benchmark
- Towse, A. et al. Health Policy 121, 1025–1030 (2017).
- Kuo, Y.-T. et al. Lancet Gastroenterol. Hepatol. 2, 707–715 (2017)
- Roope, L. S. J. et al. Science 364, eaau4679 (2019).
- Sharma, P. & Towse, A. New Drugs to Tackle Antimicrobial Resistance: Analysis of EU Policy Options (OHE, 2014).
- Projan, S. J. et al. Curr. Opin. Microbiol. 6, 427–430 (2003).
- Stokes, J. M. et al. Cell 180, 688–702 (2020).
- Enne, V. I., Livermore, D. M., Stephens, P. & Hall, L. M. C. Lancet 357, 1325–1328 (2001).
- Sundqvist, M. et al. J. Antimicrob. Chemotherpy 65, 350– 360 (2010).
- Schneider, M., Harrison, N. R., Daniel, G. W. & McClellan, M. B. Delinking US Antibiotic Payments through a Subscription Model in Medicare (Duke-Margolis Center for Health Policy, 2020).
- https://www.there100.org/re100-members
- https://www.nature.com/articles/d41586-020-02884-3
- https://www.nature.com/articles/d41586-020-02885-2
- https://insights.nordea.com/en/sustainability/engaging-the-drug-industry-on-water-pollution-in-india/
- https://www.nordea.com/en/press-and-news/news-and-press-releases/Industry-engagement-in-India-paying-off.html
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7296207/
- https://accesstomedicinefoundation.org/amr-benchmark/results
- https://www.sanofi.com/-/media/project/one-sanofi-web/websites/global/sanofi-com/home/common/docs/download-center/green_chemistry_2018.pdf/
- https://www.nature.com/articles/d41586-020-02884-3
- https://science.sciencemag.org/content/360/6390/733/tab-figures-data

